QuoData contributes to Joint Action SHARP (Strengthened International Health Regulations and Preparedness in the EU)
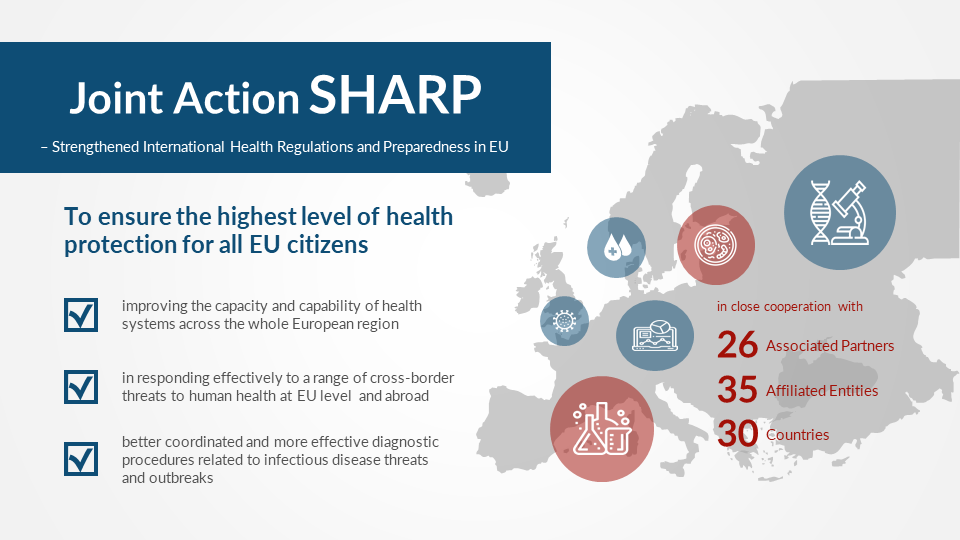
In order to ensure the highest level of health protection for all EU citizens, an EU funded Joint Action for health security has been launched. Entitled Joint Action SHARP (Strengthened International Health Regulations and Preparedness in the EU), the project is coordinated by the Finnish Institute for Health and Welfare (THL), Finland, and co-coordinated by the Robert Koch-Institut (RKI), Germany. It is carried out with 26 Associated Partners and 35 Affiliated Entities from 30 countries in close cooperation with ECDC and WHO.
The aim of SHARP is to improve the capacity and capability of health systems across the whole European region in responding effectively to a range of cross-border threats to human health such as infectious disease outbreaks at EU level and abroad.
In the framework of SHARP a European network of public health institutes focusses on highly pathogenic bacteria and viruses. By working together, all participating countries will strengthen their capacity so that they can prevent, detect and respond to possible future threats to human health.
QuoData GmbH is pleased to have the opportunity to contribute to such an important project and to support the SHARP partners. On behalf of Robert Koch-Institut, coordinator of the SHARP laboratory network, QuoData provides its expertise in the field of statistical planning and evaluation of EQAs to realize six studies on high threat bacteria and viruses.
With the overall goal of enabling an even better coordinated and more effective diagnostic procedures related to infectious disease threats and outbreaks within and outside Europe, proficiency tests play an important role in comparing the performance of SHARP partners in the field of analysis of highly pathogenic bacteria and viruses.
QuoData provides an online data entry for the participating laboratories and develops individual concepts for the comprehensive evaluation of six interlaboratory studies, including both quantitative and qualitative methods. Decisive aspects of analysis such as information on sample transport, rate of diagnosis and methods used are included in the evaluation concept. This enables an extended laboratory assessment, the specific optimization of procedures and ensures a high quality of the analysis results. For more information please visit here: https://www.sharpja.eu/
